- Introduction
Rechargeable batteries or secondary cells are batteries that can be used many times. Rechargeable batteries are an energy storage device that can be recharged after being discharged by applying DC current to its terminals. In general, rechargeable batteries are a reasonable alternative to non-rechargeable batteries or primary batteries. Not being required to be replaced in the short term and the ability to use it many times, although the consumer price of these batteries may seem higher than the original batteries at first, but in the long run, this type of battery will be more economical to consumers.
2- Features of rechargeable batteries
Among the features of rechargeable batteries, the following can be mentioned:
- Very long life
- Less damage to the environment due to the production of less waste
- Compatibility of its metal hydride type with the environment due to the absence of toxic substances inside
- High variety in size and dimensions
- Can be used in all kinds of household appliances
3- Types of rechargeable batteries
Rechargeable batteries are divided into several categories in terms of internal structure and materials used in it:
1- Nickel cadmium
2- Nickel metal hydride
3- Lithium ion
4- Lithium polymer
5- Lead acid
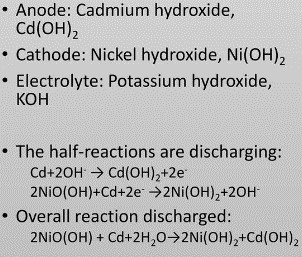
3-1 Nickel Cadmium (NiCd):
Nickel Cadmium batteries are a type of rechargeable batteries that use nickel and cadmium oxide in their structure.
The components of this type of battery are:
- A positive electrode plate of nickel (III) oxide-hydroxide.
- Negative cadmium electrode plate
- A separator
- An alkaline electrolyte (potassium hydroxide).
The most common size of rechargeable batteries that have this chemical structure is the size of AA and AAA. The most common use of these types of batteries is in household appliances such as toys, rechargeable brooms and wireless phones.
The nominal voltage of this type of battery is 1.2 volts. The lower nominal voltage compared to primary cells of carbon-zinc and alkaline cells (the nominal voltage of alkaline and carbon-zinc cells is 1.5) makes it impossible to replace primary cells in all cases of use with this type of cell.
It should be noted that cadmium is included in the category of heavy metals, and this makes disposal of these types of batteries a more complicated and difficult process than primary batteries. Cadmium is considered as a heavy metal, a toxic and dangerous metal for the environment, and its disposal or burning causes a lot of pollution. Today, many countries are looking to recycle these types of cells, but in general, due to the dangers caused by the use of cadmium, this type of cell has been replaced with nickel metal hydride technology.
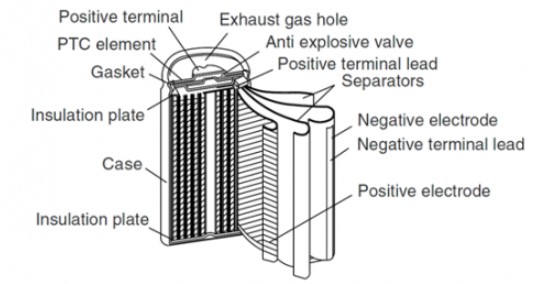
3-2 Nickel Metal Hydride (NiMH):
These batteries are very similar to the nickel cadmium type, but the removal of cadmium from the structure of these cells made the nickel metal hydride type much less harmful than nickel cadmium.
In these types of cells, the positive electrode is made of nickel oxide hydroxide and the negative electrode is a metal alloy. This alloy must have the hydrogen absorbant. During charging, electrons flow from the negative electrode to the positive electrode and attract hydrogen to the positive electrode. During discharge, electrons flow from the positive electrode to the negative electrode and release hydrogen from the positive electrode.
One of the advantages of replacing cadmium with a metal alloy was increasing the capacity of this cell by 2 to 3 times. In addition, the life of these cells increased and reached more than 1000 charge and discharge cycles.
The chemical reaction that occurs in the negative electrode of the NiMH cell is as follows:
H2O + M + e− ⇌ OH− + MH
And the chemical reaction in the positive electrode is as follows:
Ni(OH)2 + OH− ⇌ NiO(OH) + H2O + e−
During charging, reactions occur from left to right, and during discharge, the same reactions proceed from right to left. The bidirectional nature of the reactions has given secondary cells the ability to be charged.
The electrolyte of these types of cells is alkaline and potassium hydroxide is usually used as an electrolyte.
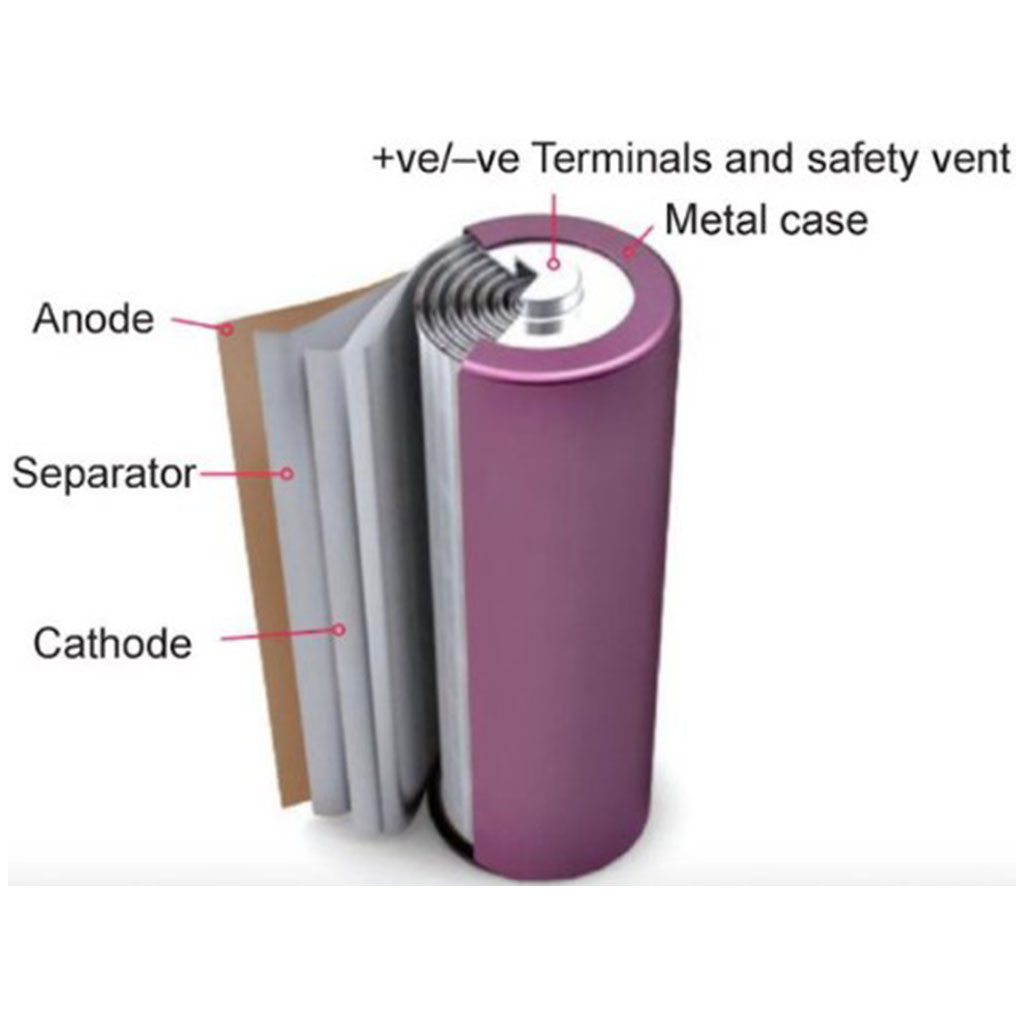
3-3 Lithium-ion:
The invention and commercialization of lithium-ion batteries may have one of the biggest impacts in recent technology history.
A lithium-ion battery is a type of rechargeable battery that uses the reversible combination of Li+ ions in electronic conductive solids to store energy. Compared to other commercial rechargeable batteries, Li-ion batteries have higher energy density, higher energy efficiency, longer life cycle and longer shelf life in packaging.
Like all batteries, these types of batteries are made of three main parts: positive electrode, negative electrode and electrolyte.
In general, the negative electrode of a typical lithium-ion cell is graphite made of carbon. The positive electrode is usually a metal oxide or phosphate. The electrolyte is a lithium salt in an organic solvent. The negative electrode (which is the anode when the cell is discharging) and the positive electrode (which is the cathode when the cell is discharging) are protected from short circuit by the separator. When the cell is being charged, the negative and positive electrodes switch their electrochemical roles (anode and cathode). However, in battery design terms, the negative electrode of a rechargeable cell is often just called the “anode” and the positive electrode the “cathode”.
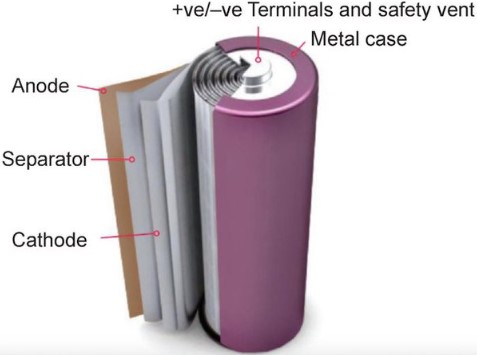
Depending on the type of materials used in lithium batteries, the voltage, energy density, lifespan, and safety can vary significantly. Currently, efforts to improve the performance of these batteries using nanotechnology are ongoing. Areas working on this are: materials used in nanoscale electrodes and other alternative electrode structures.
Lithium batteries are much more expensive than nickel-cadmium batteries, but they operate in a higher temperature range and have a higher energy density. These batteries need a protection circuit to control the peak voltage.
Batteries gradually and over time, their charge level will decrease by itself. For today’s lithium batteries, this is usually 1.5-2% per month. This rate can increase with the increase in temperature and battery charge status.
3-4 Lithium polymers:
Lithium polymer batteries or more precisely lithium ion polymer batteries are another category of rechargeable batteries that have evolved from lithium ion and lithium metal batteries. Highly conductive semi-solid polymers (gels) form this type of batteries’ electrolyte. These batteries provide higher specific energy than other types of lithium batteries.
The main difference is that instead of using a liquid lithium salt electrolyte (such as lithium hexafluorophosphate, LiPF6) that is kept in an organic solvent (such as EC/DMC/DEC), the battery uses a solid polymer electrolyte (SPE) such as polyethylene. glycol (PEG), polyacrylonitrile (PAN), poly(methyl methacrylate) (PMMA) or poly(vinylidene fluoride) (PVdF).
The basis of operation of lithium polymer batteries, like lithium ion batteries, is based on the removal of lithium ions from a positive electrode material and a negative electrode material. To prevent the electrodes from coming into direct contact with each other, a micro porous separator is placed in between, allowing only ions and not electrode particles to migrate from one side to the other.
Polymer electrolytes can be divided into two major categories: solid dry polymer electrolytes (SPE) and gel polymer electrolytes (GPE).
Compared with liquid electrolytes and solid organic electrolytes, polymer electrolyte offers advantages such as increased resistance to volume changes of electrodes during charge and discharge processes, improved safety characteristics. They also have excellent flexibility and process ability. Lithium polymer cells allow the manufacturers to take any desired shape and can be used for a wide variety of applications.
The rated voltage of this type is around 3.6 or 3.7 volts, but it is considered fully charged from 4.2 volts, and when the voltage drops to 3 to 2.7 volts, the battery is considered fully discharged. The range of application of this technology includes rechargeable batteries from household uses such as toys and small helicopters, batteries for mobile phones, tablets and laptops to larger uses such as batteries for hybrid cars. Lithium-ion batteries are becoming increasingly common in uninterruptible power supply (UPS) systems. They offer countless advantages over the traditional VRLA battery, and as stability and safety improve, trust in this technology is increasing.
Their power-to-size-to-weight ratio is considered as a huge advantage in many industries that require critical power backup, including data centers where space is often at a premium.
3-5 lead acid:
Lead acid batteries are among the batteries that are usually used for high power supply. This battery is mainly used in cars. Lead-acid batteries have large dimensions and relatively high weight. This type of battery is also used in emergency power sources due to its ability to store high energy.
Lead acid batteries are mainly divided into two categories:
1- wet lead acid batteries
2- Dry lead acid batteries
Lead acid batteries were more common in the past and are not used much now. Most new equipment, especially some types of UPS, are dry acid shielded.
Components of lead acid batteries:
- Plastic door and wall covering its outer surface.
- Plate separator that acts as an insulator.
Negative electro active plates or cathode (NAM)
Positive electro active plates or anode (PAM)
- Lead fittings
- Electrolyte
These batteries have many uses, including
- UPS power supply battery
- Telecommunication equipment battery
- Medical equipment battery
- Electric wheelchair battery
- Electric forklift battery
- Battery for solar systems
- Automatic door control circuit battery
4- Recycling of batteries:
The chemicals used in rechargeable batteries are very dangerous for the environment; hence the best way for non-consumable batteries is to recycle them.
Nickel cadmium batteries, which have very limited use today and have been replaced by nickel metal hydride batteries, are considered one of the most dangerous types of batteries for the environment. Cadmium is one of the heavy metals, which is a great danger to the environment and human health, so this type of battery was collected, in cadmium recycling centers it was recycled up to 99% and used again in other batteries.
Nickel metal hydride batteries, which have a longer life than nickel-cadmium batteries and do not pose a great risk to the environment, are recycled by mechanical methods. Plastic, hydrogen and nickel are separated from each other. After recycling, nickel is used in making stainless steel.
Lithium-ion batteries have the longest lifespan among rechargeable batteries and have a cost-effective recycling process. The recycling of these batteries is done by thermal decomposition or pyrolysis, and during this process, amounts of cobalt, iron and copper are obtained. These materials are used in iron smelting, cement factories and road construction.
Lead acid batteries also need to be recycled. In the recycling of lead-acid batteries, lead, plastic (generally polypropylene) and acid in these batteries are separated. The lead separated from the batteries is prepared in heat treatment for the production of new batteries. After washing, plastics are turned into old granules and sent to plastic factories. Their acids also return to the consumption cycle after purification.




No Comments