History of battery
Baghdadi battery or Parthian battery is considered to be the first source of storing energy. Due to discoveries associated to this type of urn, it seems that it was primarily used for plating bright metal dishes.
This special ancient type of battery was conducted of several parts that worked as a functioning unit when put together correctly. A long metal cord was placed in the middle of a clay urn and the rod was surrounded by a metal cylinder. presumably either vinegar or lemon juice was playing the role of an electrolyte by which the metal cylinder would be filled. To complete the process, the entering part of the urn was sealed by a special resin.

Before going further more through the history of batteries, one main question has to be answered: “what is a battery?”
A battery a compete functioning system which transforms the chemical energy, produced by chemical reactions, to electrical energy.
It is noteworthy to say what is known as a battery among common people and is available for purchase in supermarkets and other places is not actually a battery! using the name “battery “is a very common mistake. What we call a battery and know as battery is in fact a “cell”. A battery is consist of two or more cells.
Now that we know about the battery and a cell, it is time to learn more about the history of battery and its evolutionary process.
In 1749, Benjamin Franklin, The American scientist, connected a series of capacitors together in one of his experiments and decided to call it a battery for his own reasons. These capacitors were panels made of glass which their bigger side were sealed by metal. They could be charged by a simple generator.
Luigi Galvani was an Italian scientist who discovered the static electricity in animals. He noticed when an electrical current hits a frog’s leg, it shrinks. he believed the shrinkage happens due to accumulation of energy in the muscle.
Alessandro Volta, Italian physicist and Galvani’s friend, disagreed with Galvani and believed the frogs reaction is caused by two metal that happen to get connected with each other when a wet interference occurs. He proved his hypothesis and through experiment and published the results by 1791. In 1800, he succeeded to manufacture the first battery which later became known as “volta candle”.
The volta candle was consist of two disks, one copper and one zinc, and they were separated by a thick paper layer soaked in saltwater, the electrolyte. In contrast to “Leyden jar” , in “volta candle” the current was constantly maintained and while not used, the energy wouldn’t subside considerably.
Anyways, this primary model didn’t provide enough voltage to produce spark. After enough research and conducting multiple experiments, he finally figured the combination of zinc and silver works better than any other chosen metals and is the most officiant.
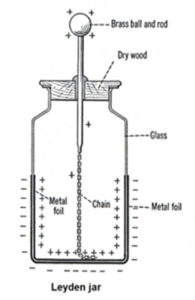
“Leyden jar” was a system designed to store electrical charge which later came to be known as the ancestor of today known capacitors. This system was used to store static electricity and separately discovered by Pieter van Musschskjenbroek in 1746 and Ewald George von Kleist in 1745.
Its primary form was a glass jar half filled with water and the opening was covered by a cork.
There was a hole in the cork and a nail was going through that hole. the sharp side had a wire connected to it that was in the water.in order to charge the glass, the end of the water was in the presence of a friction device which produced static electricity. when disconnected, touching and felling a mild shock was the way to determine if the jar is charged.
As it is shown in the figure below, the inner and outer parts of the jar are covered by metal foil. The outer part is connected to earth while a brass chain maintains the connection with the inner side which eventually comes out of the opening.
The main usage of “Leyden jar” was in classrooms as primary model of capacitor. Capacitors are widely used in radios and televisions and due to their size its hard to see the parts and analyze them so Leyden jar came to help so the students have a better comprehension of function of a “static electricity storage”.
After having a glance of the origins and history of battery, now we can see what today’s battery is consist of.
The known carbon zinc batteries or “dry cells” are the Galvanic cells which are around and used for over 140 years. Today there are two types of carbon zinc battery: first the chloride system and second Leclanché system (the latter is a French version, designed by George Leclanché in 1860). Leclanché system gained a considerable commercial attention due to usage of natural manganese dioxide (MnO2) as cathode and ammonium chloride (NH4Cl) as anode which was considerably cheap.
Later on, in 1960s, the zinc chloride technology was expanded and with better undressing of the function of stable thin separators, the sealings were improved. Also, the expanding usage of zinc chloride caused the improvement of leakage problem and wide spread use in heavy discharging.
Both types used to be popular and widespread around the world; however, nowadays the usage of this particular type of battery is descending in USA and Europe, yet we can still count it as a sufficient battery because of considerable low costs of manufacturing, available manufacturing materials, easy manufacturing process and acceptable range of use.
At last, it is worthy to mention that in 1947, the United States of America decided on a unified system of naming the batteries so they can be identified without a problem all around the world.
In this particular system that is still used, all the batteries are categorized by shape and some letters are assigned to each kind. For example, the AA is assigned to round batteries with the height of 49.5-50.5 mm and the diameter of 13.5 -14.5 mm or AAA is assigned to round batteries with the height of 43.5-44.5 mm and the diameter of 9.8 -10.5 mm etc.
These letters must be printed on the body of battery.





No Comments