Alkaline battery :
In the previous article we talked about alkaline batteries (part 1), in this article we will continue the topics.
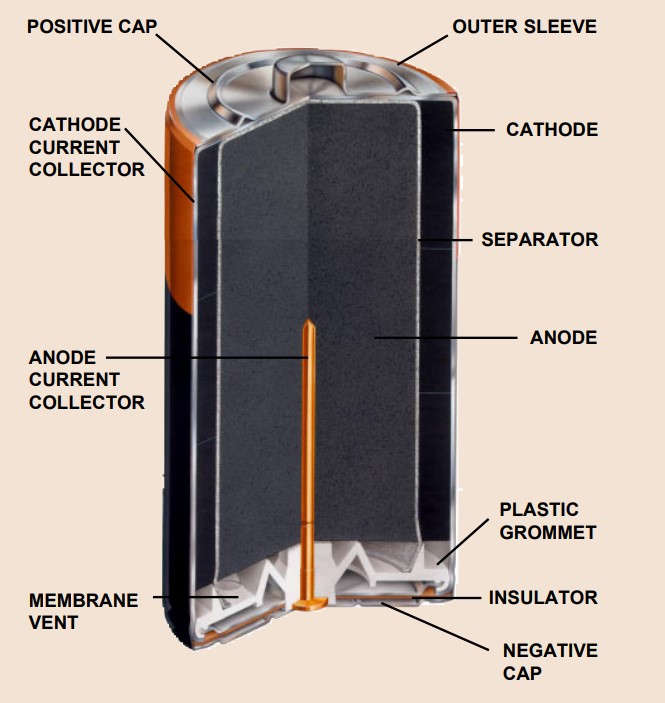
After the powder is pressed, an excellent cathode-to-cell wall contact will be formed. The steel case becomes the cathode current collector and serves as the positive terminal of the cell.
To form the anode, a precise amount of the zinc powder is dispensed into the center cavity. This procedure must happen carefully because it ensures the desired capacity and surface area.
Next, an anode current controller is placed, it must be welded to the external anode cap. It goes all the war through a plastic cap and reaches the center of the anode powder mix. The current controller must be in a close contact to the center and doesn’t change position.
The other indispensable part is the separator. The main objective of this component is to isolate the electrodes. It is a highly absorbent, ion-permeable, and chemically inert material which blocks the migration of anode particles and prevents self-discharge of the cell during periods of non-use.
For a whole battery system to work, it is essential to choose materials that are absorbent, that is why anode, cathode and the separators are porous so they can fully absorb the alkaline electrolyte solution and be saturated with it.
The structural form in contact to the alkaline electrolyte, which is highly conductive, allows the cell to perform quite well at high discharge rates and continuous service. It is also responsible for the low internal resistance and good low temperature performance.
For the cylinder to be complete and the battery we know come to completion, A plastic cap or grommet is sealed to the cell case by means of radial crimping pressure and a sealant. This resilient material ensures a tight seal to prevent loss of electrolyte. The anode cap is isolated from the positive cell case with an insulator.
A vent mechanism is incorporated into the plastic grommet to protect against cell rupture and damage in the event of misuse under abusive conditions. This vent is designed to relieve excessive gas pressure that may be generated by prolonged short-circuiting, improper disposal in fire, charging, or incorrect insertion in devices.
4.2 Multicell Construction
what we mean by multicell construction is the combination of two or more cells, placed in parallel or in series. The final manufacturing cost is lower but this type of battery is designed for specific usage and can not be used instead of a single cell battery.
The inner cells are welded using a nickel-plated steel tab material to enhances the reliability of the battery’s performance in comparison to pressure-type contacts.
4.3 Button Cell Construction
in this type, anode and cathode are in form of subassembly. There is also a separator to form a layer. The anode subassembly consists of the cell top, which is made of a bimetal laminate of nickel-plated steel and either copper or tin; a plastic grommet, used to insulate the positive and negative terminals; a pelleted zinc anode, which is placed inside the top; and an absorbent material saturated with electrolyte.
The cathode subassembly includes the cell can; the manganese dioxide cathode consolidation; and a barrier/separator, which allows current to flow but blocks any migration of material. The cathode subassembly is placed over the anode subassembly and is sealed by crimping the edge of the can over the grommet.
5-Performance Characteristics
There are some parameters by which a battery is described and be determined whether or not is standard. The most important parameters are the voltage, capacitance and internal resistance of a battery that should be thoroughly designed and examined. there are other factors such as temperature and humidity that effects the performance of a battery.
5-1 voltage:
the nominal voltage of a typical alkaline cell is 1.5 volts but the open circuit voltage can vary between 1.5 to 1.7 volts. When reached 0.8 volts the cell is considered discharged. Operating voltage is dictated by the state-of-discharge and the actual load imposed by the equipment.
5-2 capacity:
Capacity is usually expressed in ampere-hours or milliampere-hours. In order to determine the capacity the hours that battery can work under a continuous drain is multiplied by the average current flow. Alkaline cells and batteries are available in button (45 mAh to 110 mAh) and in cylindrical (580 mAh to 15,000 mAh) configurations.
5-3 Internal Resistance:
compact construction along with highly conductive electrolyte have resulted the low internal resistance in alkaline cell, which is a desirable factor when it comes to a battery. The internal resistance is usually around 1ohm and it stays the same under load and only increases at the very end of useful life. The former is another advantage of an alkaline cell compared to a zinc carbon cell.
5-4 Effect of Temperature:
just like any other product, an alkaline cell has a range of temperature under which it performs the best and the practical range for this type of cell is -4°F to 130°F (-20°C to 54°C). At lighter loads, some output can be obtained at temperatures as low as -20°F ( -30°C). up to 75 percent of the rated capacity at room temperature can be delivered at 32°F(0°C).
5-5 Type of Discharge:
there are various type of discharge for a battery and the type of discharge depends on the equipment the battery is used for. The type of discharge also impacts on the service life delivered by a battery in a specified application. Three main types of discharge are
- Constant Resistance (“R”): In this mode, the resistance of the equipment load remains constant throughout the discharge
- Constant Current (“C”): In this mode, the current drawn by the device remains constant during the discharge
- Constant Power (“P”): In this mode, the current during the discharge increases as the battery voltage decreases, thus discharging the battery at a constant power level.
5-6 Shelf Life:
shelf life means the amount of time a battery can stay in the packaging and not be used but remains practical after the packaging is opened and the battery is underload. Alkaline cells have long shelf storage life. After one year of storage at room temperature, cells will provide 93 to 96 percent of initial capacity. When stored for four years at 70°F (21°C), service of about 85 percent is still attainable. Storage at high temperatures and high humidity will accelerate degradation of chemical cells. At low temperature storage, the chemical activity is retarded and capacity is not greatly affected. Recommended storage conditions are 50°F (10°C) to 77°F (25°C) with no more than 65 percent relative humidity.
6- Applications:
with all the beneficiary characteristics mentioned before such as superior drain rate characteristics, good shelf storage life, low internal resistance and wide operating temperature range, alkaline cells have become one the most popular customers choice and come in handy for many applications from home to healthcare, industrial instruments or even specific and general military equipment. This type of cells than provide energy for:
- photographic equipment,
- remote control devices
- toys
- electronic games
- flashlights
- tape recorders
- home health care devices,
- radios
- shavers
- clocks
- calculators and computers
- portable medical and industrial instrumentation
- portable and emergency lighting products
- communications equipment
- and portable electrical measurement devices
- …..
7-battery care
To expand the life of a battery, there are some factors that should be taken into account. First and foremost, the temperature of the place in which the batteries are kept in should be between 10 °C to 25 °C. Excessive temperature cycling and storage at temperatures greater than 77°F (25°C) should be avoided to maximize shelf life.
Discharged batteries should be removed from the equipment. Also, if a device is not going to be used for a long period of time (for example a couple of months), it is highly recommended to remove the batteries to prevent any possible self-discharge or leakage.
For equipment that work with both AC current and batteries, remember to remove the batteries while it is being powered by household (AC) current.
Always replace all batteries at the same time since batteries in series, in different states of discharge, may eventually drive the weakest battery into voltage reversal with progressive risk of leak age or rupture.
Mixing battery systems, such as alkaline with zinc-carbon, may also result in voltage reversal and should be avoided.
Always replace the battery or batteries in your equipment with the size and type of battery specified by the equipment manufacturer.
Never put nonchargeable batteries in a charger.
Keep the batteries away from children. If swallowed, it can be hazardous. Consult with a doctor immediately.
8- Disposal
There aren’t any specific regulations for battery disposal but there are still actions that can be taken to preserve the natural environment. the following is recommended for the disposal of alkaline-manganese dioxide cells and batteries.
- Batteries in Household Use: Individual alkaline-manganese dioxide cells and batteries can be disposed of with other household waste.
- Commercial Quantities: The recommended procedure for disposal of alkaline-manganese dioxide cells and batteries is in a hazardous waste landfill. Since these cells are not classified as a “hazardous waste”, they can be shipped to the hazardous waste facility as a “non-hazardous” waste.


No Comments